Understanding & Maintaining
Part 1 | Introduction, Background & Terms
The knowledge assessment test will award you 2 hours of NY State CE credit. A certificate will be emailed to you upon successful completion.
Understanding & Maintaining Controlled Substance Compliance
When clinicians and LVT’s utilize and prescribe controlled substances within a facility, they must be aware of and adhere to the Federal and State regulations and policies that govern their use. This workshop is designed as an introduction to these regulations and policies, as well as to the paperwork and record keeping required for the use of controlled substances.
The objectives of this training are to provide:
- An understanding of the Federal and State regulations for using controlled substances.
- An understanding of the policy for using controlled substances.
- A detailed description of record-keeping requirements for using controlled substances.
- A resource for personnel about the forms, instruction documents and other information available to help simplify compliance with the regulations and policy.
Background
A controlled substance is a drug or chemical whose manufacture, possession and use are regulated by the Federal and State government. This may include illegal drugs and prescription medications.
Controlled drugs are rated in the order of their abuse risk and placed in Schedules by the Federal Drug Enforcement Administration (DEA). The drugs with the highest abuse potential are placed in Schedule I, and those with the lowest abuse potential are in Schedule V. These schedules are commonly designated as C-I, C-II, C-III, C-IV, and C-V.
The mission of the Drug Enforcement Administration (DEA) is to enforce the controlled substances laws and regulations of the United States. The DEA is responsible for suppressing illegal drug use and distribution by enforcing the Controlled Substances Act. The DEA requires a registration for the possession and use of controlled substances. For more information on the DEA, go to http://www.usdoj.gov/dea/index.htm.
The New York Board of Pharmacy (NYBP) is the agency authorized by NY statute to regulate controlled substances in the State of NY. The NYBP exists to protect the public from adulterated, misbranded and illicit drugs, and from unethical or unprofessional conduct on the part of pharmacists or other licensees. The NYBP requires researchers using controlled substances to obtain an NYBP registration certificate. For more information on the NYBP go to http://www.op.nysed.gov/prof/pharm/.
Terms used in the policy
The DEA policy for, Using Controlled Substances, contains several unique terms that form the structure of the policy:
For each of these terms, think about how it applies in your facility.
Unit: A department or other administrative structure, like a division or institute.
Location: A room or designated area where controlled substances are stored in a safe.
Unit Registrant: A person who is appointed by the administrator to hold the DEA and NYSBP registrations for the Unit, exercises signature authority and approves or appoints the Location Registrants for the Unit. Also referred to as the DEA registrant.
Location Registrant: A person, usually the principal investigator (PI) or lab supervisor, who obtains an NYSBP registration for the (lab) Location, supervises the Authorized Users and assures compliance with the controlled substances policy and regulations at the Location.
Authorized Users: All personnel who perform one or more activities (receiving, using, disposing, etc.) with research controlled substances under supervision of a Location Registrant. Authorized Users are not required to have a specific registration. Authorized Users must receive training for lab-specific use of controlled substances.
Standard Operating Procedure
Controlled substances must be properly handled at all stages of purchase, use, storage, transfer and disposal. The DEA has a Standard Operating Procedure (SOP), that can serve as a reference. We recommend that you bookmark the SOP for future reference. The SOP defines the person who is responsible for each of the activities related to using controlled substances.
Part 2 | Registering, Purchasing, Receiving, Labeling, Storing & Securing
Registering for Controlled Substances
The DEA requires a registration for different types of activities when using controlled substances, e.g., research, teaching, clinical practice and other activities.
Unit Registrants and Location Registrants must understand the Unit and Location structure, registration process, roles and responsibilities.
Authorized Users do not need a specific registration.
Everyone performing any activity with controlled substances must sign the Authorized Users Signature Log. The Authorized Users Signature Log is one of the documents required by DEA policy that must be kept up-to-date and filed in your controlled substances records.
Authorized Users Signature Log

The Authorized User Signature Log, like all controlled substance records, must be kept for a minimum of seven years. When a DVM departs the facility, note the date the person left on the form. If there is high turnover in your facility, you may need to start a new log, but remember to keep the original document with your controlled substance records.
Purchasing Controlled Substances
The DEA policy has specific procedures to be followed when purchasing controlled substances:
- The Unit Registrant must be aware of all purchases of controlled substances. Ensure this by providing an initialed and dated copy of the purchase receipt to the Unit Registrant.
- File the purchase receipt with the Location controlled substances records.
- Purchasing controlled substances from centralized sources is highly recommended.
- To pick up controlled substances, pharmacies require a copy of the DEA registration, the Authorized Users Signature Log and photo identification.
The purchase of C-I and C-II substances require completion of a DEA Form 222.
DEA Form 222 Order Forms
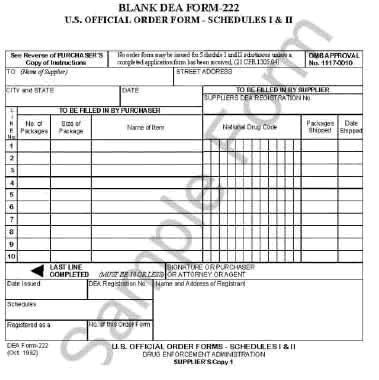
After the DEA has approved your use of C-I or C-II controlled substances, they will send you the DEA Forms 222. After initial approval, you may request additional Forms 222 online from the DEA website.
Follow the guidance provided in “Using DEA Form 222 to Order Controlled Substances,” http://www.health.state.ny.us/professionals/narcotic/laws_and_regulations.htm.
Receiving Controlled Substances
When receiving controlled substances, or if controlled substances are shipped to your facility, ensure that, the controlled substances are processed as soon as possible, by:
- Recording receipt of substances in the disposition record.
- Locking controlled substances in the safe immediately.
In addition, ensure that the purchase records meet the DEA regulations by:
- Initialing and dating purchase receipt.
- Providing a copy for Unit Registrant’s records.
- Filing the purchase records with the controlled substances records.
Labeling Controlled Substances
All containers of controlled substances must be properly labeled. If a facility re-packages, compounds or dilutes controlled substances, appropriately label the re-packaged, compounded or diluted substance and store it in the safe.
The label on diluted or combined controlled substances that will be stored in the safe must include the following information:
- Name of controlled substance.
- Schedule of drug.
- Lot number.
- Final strength or concentration of controlled substance.
- Volume or amount of substance per container.
- Date of dilution and initials/expiration date no more than 30 days after dilution.
Storing and Securing Controlled Substances
Controlled substances must be stored securely, according to the DEA regulations. The following points highlight the requirements:
- Store controlled substances in a locked safe bolted to an immovable object.
- Store controlled substances separately from other drugs and materials.
- Limit access to the safe.
- For expired drugs, mark the container “Expired” and segregate in the safe.
- Store the slurry bottle with contaminated waste controlled substances in the safe.
- Store controlled substances requiring cold storage in a refrigerator or freezer with a locked door.
Part 3 | Using, Transferring, Disposing
Using Controlled Substances
The DEA policy mandates the following about the use and record-keeping of controlled substances:
- Only Unit Registrants, Location Registrants and Authorized Users may use controlled substances.
- The person using the controlled substances must be the one to initial and date the disposition record for the particular action performed.
Record-keeping for Use of Controlled Substances
Every activity with controlled substances must be recorded on the disposition record, which is the primary record of all activities with controlled substances. The recording requirements vary by the type of drug being used:
- For C-III, C-IV and C-V drugs, the total volume used on a daily basis may be recorded on the disposition record. For instance, if ten animals were given 0.3 ml of a substance on a specific day, the total in the disposition record could read 3.0 ml. However, the individual use must be recorded in a surgical, anesthetic or other medical record that is accessible.
- The regulations for use of C-II controlled substances (e.g. pentobarbital) are more stringent. Each individual dose must be recorded in the disposition record.
- In the instance of an animal surgery, the individual recorded dose can be the total per animal per anesthetic episode. If an animal was given 1.0 ml of a substance and boosted with 0.5 ml three times during the surgery, the total individual dose would be recorded as 2.5 ml.
- The DEA requires the use of a Controlled Substance Disposition Record that contains all the required elements. A template is available on the DEA website for reference at: http://www.health.state.ny.us/professionals/narcotic/laws_and_regulations.htm. A modified form may be used, as long as it possesses all required elements.
Note that the disposition records are dedicated exclusively to tracking all controlled substances activities. The actual use of the controlled substances must be demonstrated by recording it in surgical, anesthetic, post operative or other experimental record. This would be analogous to a hospital pharmacy dispensing a drug and a clinician writing the use of the drug in the medical record.
Example: Using the Disposition Record for C-II Controlled Substances
This example uses sodium pentobarbital, a C-II controlled substance, to demonstrate the more stringent record-keeping requirements. For a C-II controlled substance, remember that the total use for each animal must be recorded individually. For C-II drugs, do not combine the total use per day in multiple animals, as you can for C-III through C-V drugs.
The disposition record is used to track all activities:
- Receiving.
- Using.
- Diluting or combining.
- Disposing of.
- Transferring.
This section will introduce the form, and demonstrate how each activity is recorded.
Controlled Substances Disposition Record
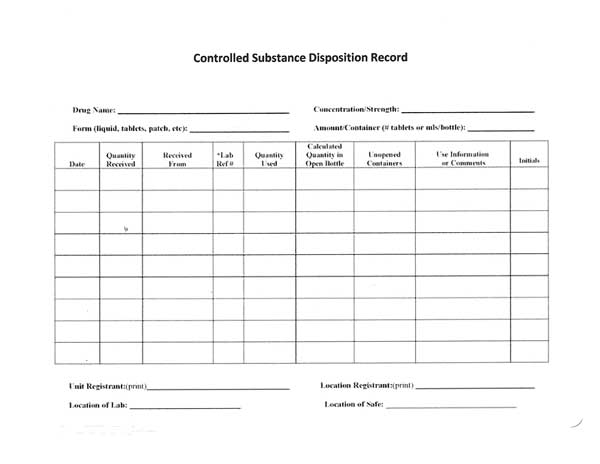
The recommended disposition record contains the required elements from the DEA regulations, which are included at the top and bottom of the form:
- Name of controlled substance
- Concentration or strength
- Form or type
- Amount per container
- Unit (DEA) and Location Registrant’s names
- Location
- Sequential page numbers
*Date and initial each activity.
*Cross out unused columns and lines.
Lines 2-4: Using controlled substances
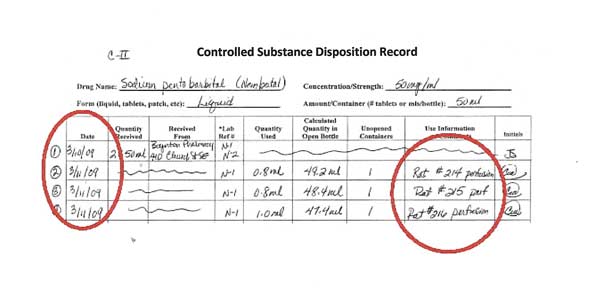
In this example, Lines 2-4 represent the use of sodium pentobarbital in three animals, each recorded individually. The first two animals, 214 and 215, received 0.8 ml of drug. 216 received 1.0 ml of sodium pentobarbital.
The lines referring to the receipt of controlled substances are crossed out.
Each activity is dated and initialed by the person using the controlled substance.
In the “Use Information” column, write enough specific information so that you can go to the surgical sheet where that use of the controlled substance is recorded. Writing only “canine” or “feline” Is not enough information when using controlled substances.
Line 5: Diluting or combining controlled substances
When controlled substances are diluted or combined, it must be recorded on the disposition record. When a drug combination is used, track the combination use separately from the stock bottle use.
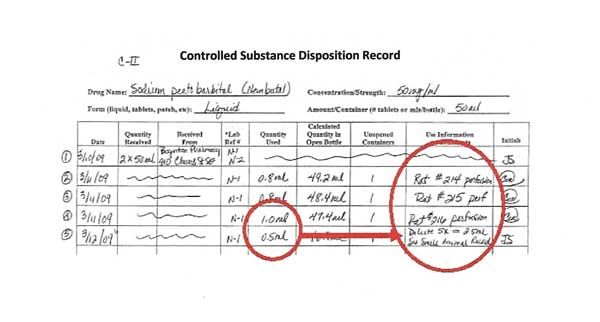
In Line 5 of the disposition record, 0.5 ml of sodium pentobarbital is used from the stock bottle and diluted 5-fold to make 2.5 ml total volume to use in mice.
When diluting (as in this example using sodium pentobarbital) or combining drugs (when using ketamine-xylazine or other drug combinations), it is necessary to record the amount used from the stock bottle(s) AND to record the amount of diluted or combined drugs used.
A sterile container and pharmaceutical grade diluents must be used when diluting or combining controlled substances for use in animals. The diluted or combined controlled substance expires after 30 days.
In Line 5, the 0.5 ml is the stock bottle amount used. The 2.5 ml of diluted sodium pentobarbital (new concentration of 10 mg/ml) is the diluted amount of controlled substances that must also be tracked.
In the example, this 2.5 ml diluted sodium pentobarbital will also be recorded on the Controlled Substances Single Drug Disposition Record.
Controlled Substances Single Drug Disposition Record
Using the Single Drug Disposition Record is convenient when multiple animals are logged and/or when other information, e.g., animal weight, is needed. It is important to remember that with a C-II controlled substance like sodium pentobarbital you are required to record the drug use for each animal separately along with all patients.
Complete the required information at the top of the Controlled Substances Single Drug Disposition Record.
In the body of the record, the columns on the left side pertain to use of the controlled substance and the columns on the right side could be used to record information relevant to your research.
In this example with diluted sodium pentobarbital, each of 10 mice received 0.2 ml, which leaves 0.5 ml diluted sodium pentobarbital remaining.
As noted on this disposition form, the 0.5 ml was put in the slurry bottle for disposal. The volume placed in the slurry bottle must also be recorded on the disposal form. More information on the use of the disposal form and slurry bottle is presented below.
When large numbers of animals are used and the information is the same (e.g. euthanizing a large number of animals with the same dosage of drug), this record could be prefilled on the Single Drug Disposition Record which is an Excel spreadsheet. If this is done, the record must be printed and signed or initialed by the person performing the activity.
In addition, to save time or minimize duplication of recording, researchers may choose to use the Controlled Substances Single Drug Disposition Record as a surgical or procedure record by adding other research elements to the record. If this is done, file the original record with your research records and a copy in your controlled substances records.
If your laboratory uses only the Single Drug Disposition Record as a controlled substance record, it is very important to clearly record all the controlled substances activities (receiving, using, diluting or combining, disposing and transferring) on the form.
By recording the amount you put in the slurry bottle on both the disposition record and disposal record, you can easily track the controlled substance. More information on disposal is provided below.
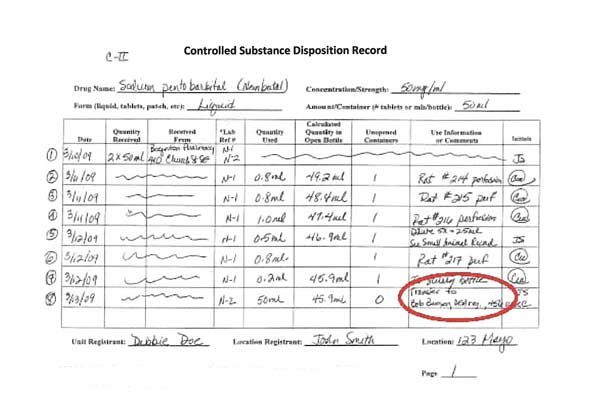
Line 8: Transferring controlled substances
Although it is not advised, it is possible to transfer controlled substances to another DEA registration. Only transfer unopened bottles of controlled substances so that both parties are certain of the amount being transferred. Both the receiving and the supplying laboratories must record the transfer in their disposition records.
In this example, Line 8 records the transfer of one 50 ml bottle of sodium pentobarbital to another laboratory. The receiving laboratory must record the 50 ml bottle in their disposition records, who they received it from and the DEA number. Because sodium pentobarbital is a C-II substance, a Form 222 order form must be used to make the transfer.
More instructions are provided in “Using DEA Form 222 to Order Controlled Substances,” http://www.health.state.ny.us/professionals/narcotic/laws_and_regulations.htm


